Abstract
Myelofibrosis (MF) is a stem cell-derived hematopoietic malignancy characterized by constitutive activation of JAK/STAT signaling through mutations in JAK2, calreticulin or MPL. MF patients have reduced life expectancy due to cytopenias, progression to acute myeloid leukemia and thromboembolic events. JAK kinase inhibitors such as ruxolitinib reduce constitutive symptoms but are not curative, with persistence of malignant cells. Allogeneic stem cell transplant remains the only curative therapy for MF, but is associated with considerable morbidity and mortality, defining an urgent need for new therapies that induce more profound and durable responses.
We used a barcoded lentiviral shRNA library to identify survival-critical genes for MF in HEL cells (homozygous for JAK2V617F), established from a patient with erythroleukemia. shRNAs targeting RAN and binding protein (RANBP) were significantly depleted, implicating nucleocytoplasmic transport (NCT) as one of the top survival-critical pathways. RAN is a small GTPase, whose cycling between GTP- and GDP-bound forms provides the engine for NCT. We previously presented results showing that shRNA-mediated knockdown of RAN, significantly reduced proliferation and induced apoptosis in HEL and SET-2 cells (harboring heterozygous JAK2V617F). Additionally, KPT-330 (selinexor, Karyopharm Therapeutics), an inhibitor of CRM1, the core component of NCT, also inhibited proliferation of HEL and SET-2 cells and colony formation by primary MF CD34+ cells, but not cord blood (CB). Importantly, inhibition of NCT was effective at reducing colony formation in samples from ruxolitinib-refractory MF patients, regardless of mutation status. A clinical trial to evaluate KPT-330 in ruxolitinib-refractory MF is in preparation.
KPT-8602 is a second-generation inhibitor of CRM-1. Unlike KPT-330, KPT-8602 does not cross the brain-blood barrier, resulting in minimal anorexia, malaise, and weight loss [Etchin et al., Leukemia, 2017 Jan;31(1):143-150]. To determine the efficacy of KPT-8602 in MF, we tested KPT-8602 in cell lines, patient samples, and a mouse model of MF, along with ruxolitinib as comparison.
In cell proliferation assays, KPT-8602 was five-fold more potent than ruxolitinib against HEL cells (IC50: 83 nM vs. 437 nM) and as potent as ruxolitinib in SET-2 cells (IC50: 95 nM vs. 85 nM). In clonogenic assays, KPT-8602 showed selectivity toward primary MF vs. CB CD34+ cells (IC50: 25 nM for MF vs. 258 nM for CB) and, in combination with ruxolitinib, resulted in a 95% reduction in colony formation. KPT-8602 inhibited colony formation by CD34+ cells from all MF patients tested, including ruxolitinib-refractory cases, irrespective of mutation status (including triple negative).
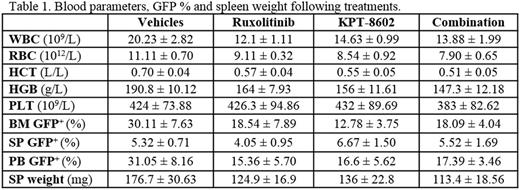
To evaluate the possible use of KPT-8602 in vivo, we used a mouse transduction/transplantation model of JAK2V617F - positive myeloproliferative neoplasm (MPN). In this model, mice develop MPN resembling polycythemia vera that progresses to MF [Bumm et al., Cancer Res., 2006, 1;66(23):11156-65]. MPN was evident based on elevated blood counts and blood GFP+ cells (representing JAK2V617F allelic burden). MPN mice were randomized into four groups: vehicles, KPT-8602 (initial dose 15 mg/kg daily, orally), ruxolitinib (initial dose 50 mg/kg twice daily, orally) or combined ruxolitinib with KPT-8602 at these same concentrations, and treated for 28 days. KPT-8602 as a single agent reduced red blood cells (RBC), white blood cells (WBC), BM GFP+ cells, peripheral blood (PB) GFP+ cells and spleen size to the same extent as ruxolitinib, and the combination was no more effective than either single agent (Table 1). Spleen cytoarchitecture was fully restored by KPT-8602, but bone marrow fibrosis was not rectified in any treatment group. Mice in all groups, including the combined vehicle controls, still experienced weight loss and lethality, suggesting that toxicity may be due to vehicles, and that optimization of drug formulations and dosing is warranted.
Overall, these results indicate that NCT is critical for MF survival, and that NCT inhibition may provide therapeutic benefit for MF patients. KPT-8602 is being tested in a clinical trial (NCT02649790) for refractory multiple myeloma. The results of our study provide strong rationale for a clinical trial in patients with refractory MF.
Baloglu: Karyopharm Therapeutics Inc: Employment, Equity Ownership. Shacham: Karyopharm Therapeutics Inc: Employment, Equity Ownership, Membership on an entity's Board of Directors or advisory committees. Deininger: Ariad Pharmaceuticals, Bristol Myers Squibb, CTI BioPharma Corp, Gilead, Incyte, Novartis, Pfizer, Celgene, Blue Print, Galena: Consultancy, Membership on an entity's Board of Directors or advisory committees; Novartis: Consultancy, Research Funding; Incyte: Consultancy; Pfizer: Consultancy; Celgene: Research Funding; ARIAD: Consultancy; Gilead: Research Funding; BMS: Consultancy, Research Funding.
Author notes
Asterisk with author names denotes non-ASH members.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal